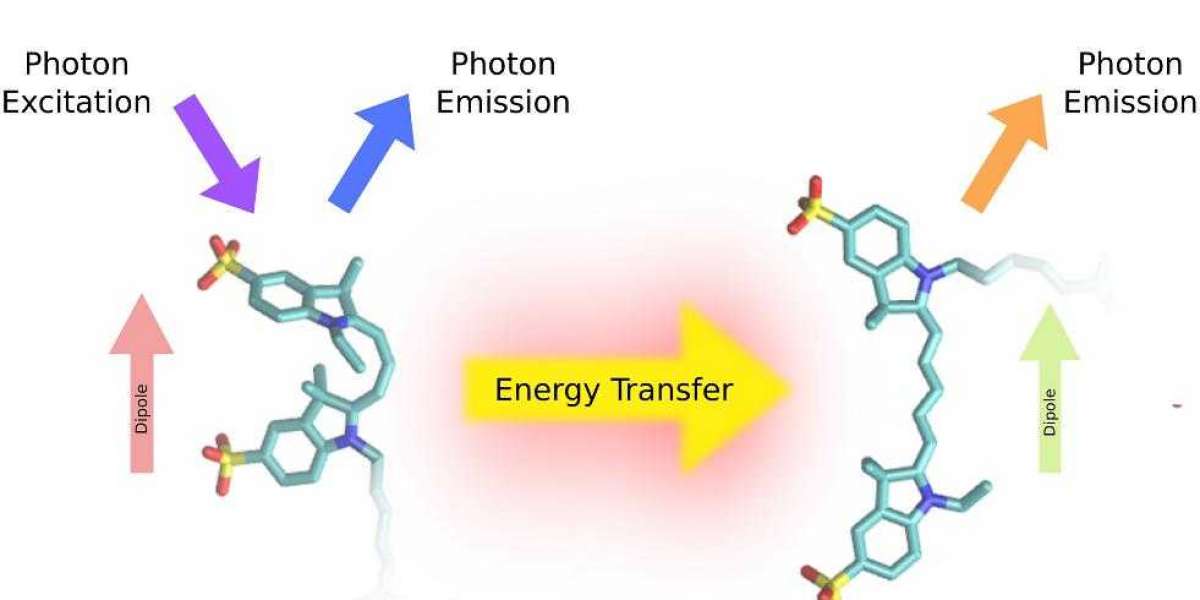
What is Fluorescence Resonance Energy Transfer?
Fluorescence Resonance Energy Transfer (FRET) has brought about a seismic shift in the realm of biological research over the past few years. This powerful and sophisticated technique is a non-radiative energy transfer mechanism, which occurs between two chromophores - typically a donor and an acceptor - when they come into close proximity. The energy transfer takes place owing to the dipole-dipole interaction between the molecules of the donor and acceptor. The technique of FRET has an extensive and widespread usage in various domains of biology, such as protein-protein interactions, DNA-protein interactions, and signaling pathways.
Fluorescence Resonance Energy Transfer Principle
The principle of energy transfer between two fluorescent molecules forms the basis of FRET. The donor fluorophore, which has a specific wavelength, absorbs light and then emits it at a longer wavelength. When the donor fluorophore comes close to an acceptor fluorophore, the energy from the excited donor fluorophore gets transferred to the acceptor fluorophore, which then emits light at a longer wavelength than the donor. The efficiency of FRET depends on the distance between the donor and acceptor fluorophores, as well as their relative orientations. This intricate and fascinating phenomenon of FRET has revolutionized the way researchers study various biological processes.
How Does Fluorescence Resonance Energy Transfer Work?
FRET can be used to detect changes in distance or conformation of molecules in a sample. To perform FRET, two fluorescent molecules, the donor and acceptor fluorophores, are chosen based on their spectral properties. The donor fluorophore is excited with light of a specific wavelength, which causes it to emit light at a longer wavelength. If the acceptor fluorophore is within a certain distance of the donor fluorophore, the energy from the excited donor fluorophore is transferred to the acceptor fluorophore, which then emits light at a longer wavelength than the donor.
The efficiency of FRET can be measured by calculating the ratio of the emission intensity of the acceptor fluorophore to the emission intensity of the donor fluorophore. This ratio, known as the FRET efficiency, is proportional to the distance between the donor and acceptor fluorophores. By measuring the FRET efficiency, it is possible to determine the distance between the two fluorophores, as well as any changes in distance or conformation of the molecules in the sample.
learn more:methods for detecting protein-rna interactions

Schematic diagrams depicting the three conditions that must be met for efficient FRET (Broussard et al. , 2013).
Single Molecule Fluorescence Resonance Energy Transfer
Single-molecule fluorescence resonance energy transfer (smFRET) is a variant of FRET. In smFRET, individual molecules are immobilized on a surface and their fluorescence properties are measured using total internal reflection fluorescence (TIRF) microscopy. This technique allows the observation of individual molecules in real time, providing unprecedented insights into the dynamics of molecular interactions.

Single-molecule FRET event (Wallace et al. , 2017)
What is Fluorescence Resonance Energy Transfer Used For?
FRET has an expansive array of applications in biological research. One of its pivotal roles is in drug discovery, where it can be used to scrutinize the interactions between drugs and their targets. By endowing drugs and their targets with donor and acceptor fluorophores, FRET can facilitate the measurement of the binding affinity of the drug to its target, as well as decipher the intricate mechanism of action of the drug.
Another significant application of FRET is in studying protein-protein interactions, which is of paramount importance in understanding various biological processes. By affixing donor and acceptor fluorophores to two proteins of interest, FRET can precisely measure the strength and dynamics of their interaction. This critical information can be harnessed to fashion new drugs that can specifically target protein-protein interactions.
FRET also holds immense potential in the field of cell biology, where it can be used to delve into the movement and localization of molecules in living cells. By grafting donor and acceptor fluorophores onto molecules, FRET can trace the real-time movement of these molecules and determine how they are transported within cells. The insight gained from these investigations can potentially pave the way for the development of novel therapies for diseases such as cancer, which involve aberrant cell movement and migration.
Moreover, FRET is a valuable tool in studying enzyme activity and signaling pathways in cells. By attaching donor and acceptor fluorophores to substrates and enzymes, FRET can effectively monitor the activity of the enzyme in real-time and investigate the dynamics of enzymatic reactions. The data obtained from these studies can be leveraged to design biosensors that can detect enzyme activity and devise new drugs that can specifically target the enzymes involved in various disease processes.
Finally, FRET can also be used to study gene expression and regulation in living cells. By grafting donor and acceptor fluorophores onto DNA or RNA molecules, FRET can track the binding of transcription factors or other regulatory molecules to specific DNA sequences in real-time. This information can be instrumental in understanding the intricate mechanisms of gene expression and regulation and can aid in the development of new therapies for genetic diseases.
References
- Broussard, Joshua A., et al. "Fluorescence resonance energy transfer microscopy as demonstrated by measuring the activation of the serine/threonine kinase Akt." Nature protocols 8.2 (2013): 265.
- Wallace, Bram, and Paul J. Atzberger. "Förster resonance energy transfer: Role of diffusion of fluorophore orientation and separation in observed shifts of FRET efficiency." PLoS One 12.5 (2017): e0177122.