Prader-Willi Syndrome (PWS) is among the most complex and burdensome rare disorders, affecting multiple systems of the body. A result of genetic abnormalities on chromosome 15, it leads to symptoms such as low muscle tone, cognitive delay, behavioral issues, and, most notably, extreme hunger (hyperphagia). For decades, families and physicians have struggled to find effective ways to manage the condition. The approval of VYKAT XR marks a pivotal moment in the effort to control the devastating impacts of hyperphagia in PWS patients.
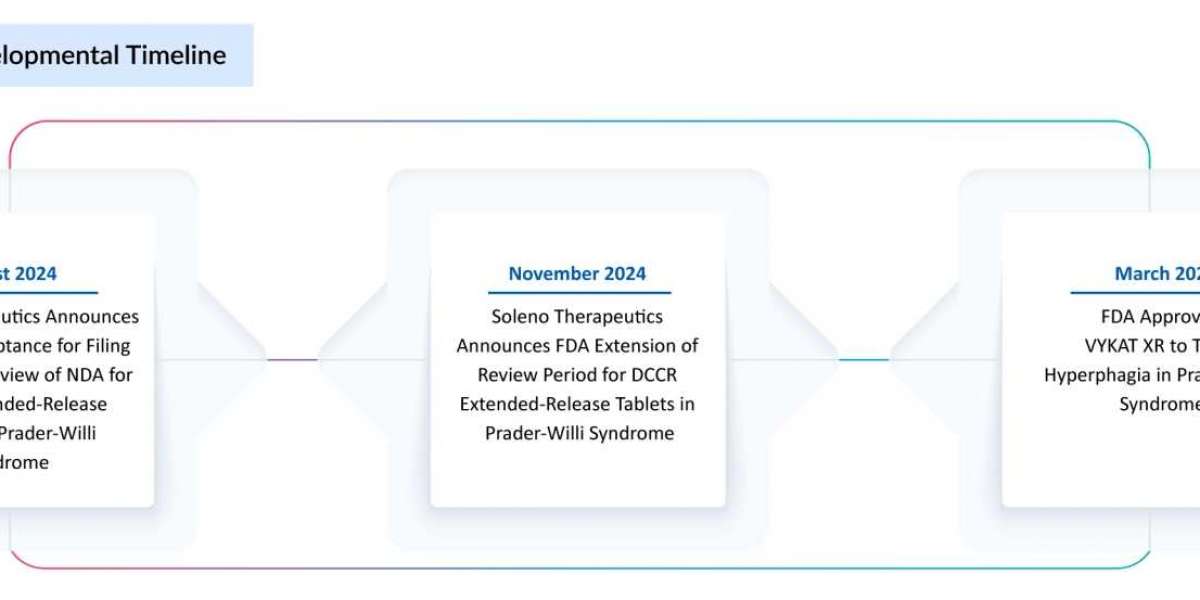
Approved by the U.S. Food and Drug Administration in 2024, Soleno Therapeutics’ VYKAT XR is the first therapy officially recognized for the treatment of hyperphagia in people with PWS. The once-daily extended-release medication offers an evidence-based, targeted approach to appetite control. This advancement elevates the current standards of Prader-Willi Syndrome treatment, which were previously limited to supportive care, hormone therapies, and off-label interventions.
Before this approval, the Prader-Willi Syndrome therapeutics market was hampered by failed trials and the absence of regulatory successes. Several promising candidates, including carbetocin and DCCR, faltered during late-stage development due to safety concerns or limited efficacy. These setbacks dampened enthusiasm and slowed investment in the rare disease space. VYKAT XR’s success changes the narrative and offers a renewed path forward.
What makes VYKAT XR unique is its mechanism, which modulates serotonin pathways involved in appetite and behavioral control. Clinical trials showed that the therapy led to a marked reduction in food-seeking behaviors and hyperphagia, providing a better quality of life for patients and easing the caregiving burden on families. This approval represents a significant leap in treating Prader-Willi Syndrome not only by reducing symptoms but also by establishing a precedent for future therapeutic innovation.
The drug’s success has reignited interest in the field, and several pharmaceutical players are reassessing their development strategies for PWS. Early-phase programs focusing on genetic therapies and novel hormonal pathways are emerging. Moreover, the ongoing collaboration between academic institutions, biotech firms, and advocacy groups is expected to push these programs into more advanced stages.
Alongside pharmacological advances, comprehensive care remains essential. Interventions such as speech therapy, behavioral counseling, growth hormone replacement, and dietary management continue to be critical for optimal outcomes. However, the integration of VYKAT XR into treatment regimens offers a much-needed layer of support in curbing excessive appetite and the behavioral issues it drives.
The broader impact of VYKAT XR extends to policy, reimbursement, and patient advocacy. Insurance coverage decisions, once hindered by the lack of FDA-approved drugs for PWS, are now shifting to accommodate this new treatment. The approval has also increased visibility for PWS among regulators and funders, setting the stage for greater investment in rare genetic disorders.
Latest Blogs Offered By DelveInsight: