Introduction: Expediting the Development of Life-Saving Therapies
The Breakthrough Therapy (BT) designation, granted by regulatory agencies like the FDA, is designed to expedite the development and review of drugs intended to treat serious or life-threatening conditions. The breakthrough therapy designation market reflects the impact of this designation on pharmaceutical development, driving innovation and patient access to novel therapies. This market is driven by the increasing need for effective treatments for unmet medical needs and the desire to accelerate the drug development process.
Mechanisms of Action: Facilitating Regulatory Pathways
The BT designation facilitates regulatory pathways by providing intensive guidance from regulatory agencies, including more frequent meetings and communication. This helps to streamline the development process and ensure that promising therapies reach patients as quickly as possible.
Applications in Oncology: Rapid Approval of Cancer Therapies
The BT designation is widely used in oncology, expediting the development and approval of cancer therapies. This includes applications for drugs targeting various cancer types, such as lung cancer, breast cancer, and leukemia.
Rare Diseases: Accelerating Development for Unmet Medical Needs
The BT designation is crucial in the development of therapies for rare diseases, where patient populations are small and unmet medical needs are high. This designation helps to accelerate the development of orphan drugs and improve access to life-saving treatments.
Neurological Disorders: Addressing Serious Conditions
The BT designation is used in the development of therapies for neurological disorders, such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis. This helps to address serious conditions with limited treatment options.
Increased Investment and Funding: Attracting Resources for Development
The BT designation often leads to increased investment and funding for drug development, attracting resources from venture capital firms, pharmaceutical companies, and government agencies. This helps to support clinical trials and accelerate the development process.
Enhanced Regulatory Support: Streamlining Review and Approval
The BT designation provides enhanced regulatory support, including priority review and accelerated approval pathways. This helps to streamline the review process and ensure that promising therapies reach patients as quickly as possible.
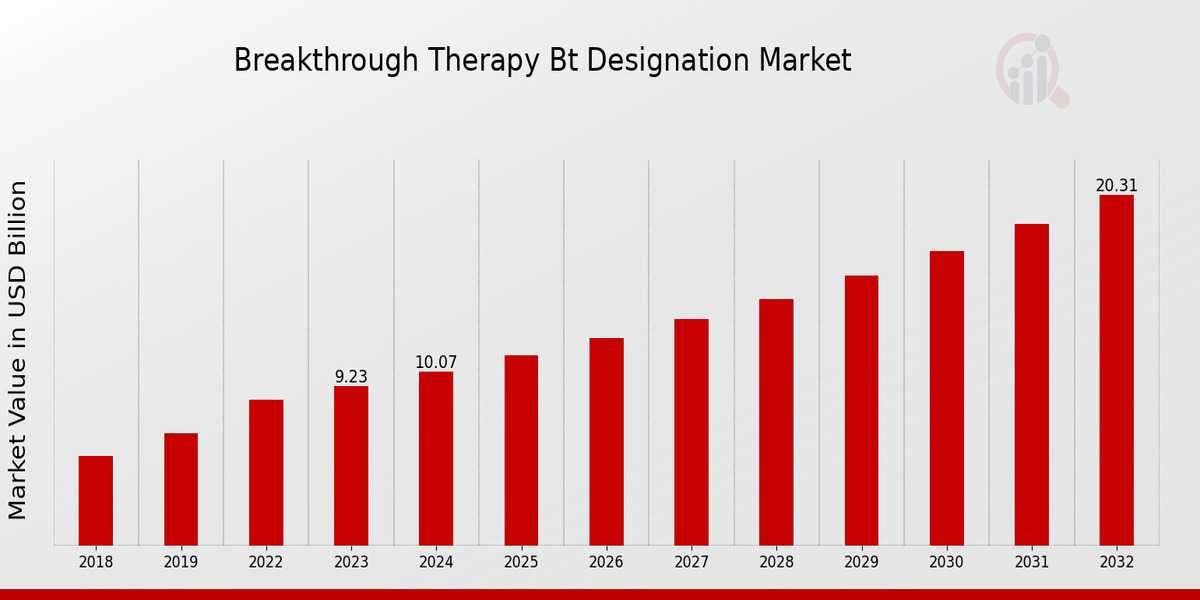
Breakthrough Therapy BT Designation Market Overview
As per MRFR analysis, the Breakthrough Therapy BT Designation Market Size was estimated at 10.99 (USD Billion) in 2024. The Breakthrough Therapy BT Designation Market Industry is expected to grow from 12.00 (USD Billion) in 2025 to 26.41 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 9.16% during the forecast period (2025 - 2034).
Improved Patient Access: Faster Access to Novel Therapies
The BT designation improves patient access to novel therapies by accelerating the development and approval process. This helps to address unmet medical needs and improve patient outcomes.
Challenges and Future Directions: Balancing Speed and Safety
Challenges in the market include the need to balance speed and safety in drug development, ensure equitable access to BT-designated therapies, and address the high cost of these treatments. Future research is focused on developing more predictive biomarkers, integrating real-world evidence into regulatory decision-making, and enhancing patient engagement in clinical trials.
Conclusion: Driving Innovation and Improving Patient Outcomes
The breakthrough therapy designation market is driving innovation and improving patient outcomes by expediting the development and approval of life-saving therapies. By facilitating regulatory pathways and attracting investment, this designation is playing a crucial role in addressing unmet medical needs. As regulatory frameworks evolve, we can expect to see even more effective approaches to accelerating drug development in the years to come.
Explore Our Latest Reports
? Androgenetic Alopecia Market
? Brain Tumor Diagnostics Market
? Ultrasound Needle Guides Market
? Pharmaceutical Membrane Filtration Market
? Stay ahead in the healthcare industry. Browse our latest insights now!
About Market Research Future (MRFR)
Market Research Future (MRFR) is a global market research firm that provides comprehensive insights into market trends, drivers, challenges, and opportunities. We offer a broad range of market intelligence reports and consulting services to help businesses and enterprises in various industries make informed decisions
Media Contact:
Market Research Future (MRFR)
Phone: +1-646-845-9312
Email: contact@marketresearchfuture.com
Website: marketresearchfuture