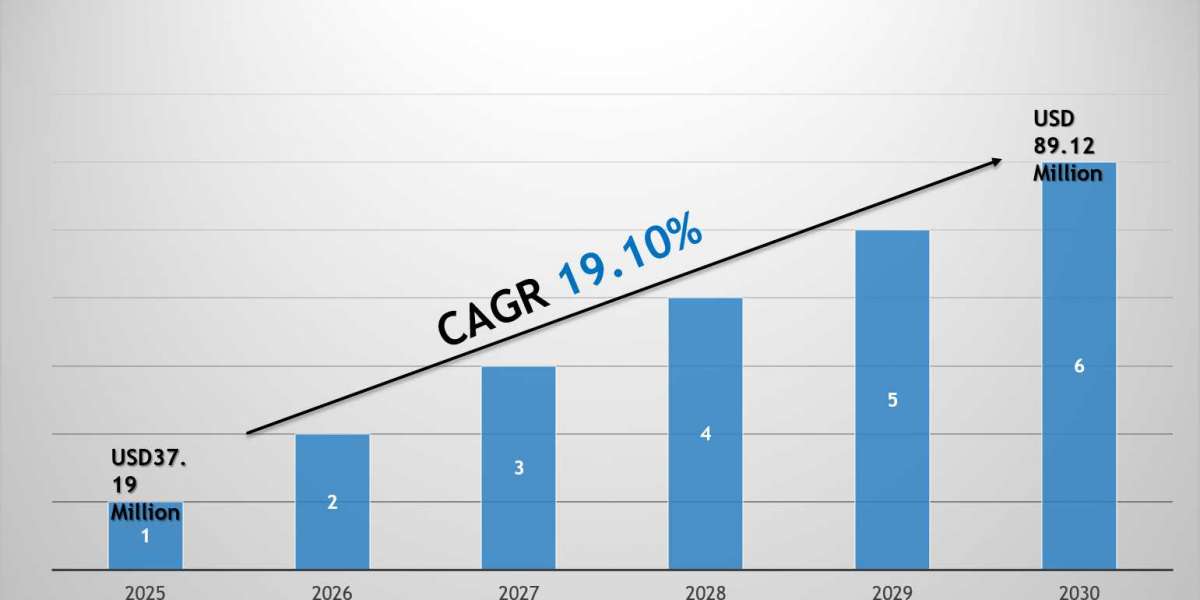
What’s fueling the sudden surge in demand for outsourced pharmaceutical ingredients?
In a world where speed, cost-efficiency, and scalability are the new rules of the game, pharmaceutical companies are rethinking how they bring drugs to market. One major shift is the increasing reliance on outsourcing the production of active ingredients to specialized partners. The Active Pharmaceutical Ingredient CDMO Market is seeing explosive growth as both big pharma and biotech firms seek flexible and cost-effective solutions without sacrificing quality or compliance.
Why are companies outsourcing what used to be a tightly controlled in-house operation?
API production is not only capital intensive but also highly complex, requiring stringent regulatory oversight and advanced technology. For many drug manufacturers, building and maintaining infrastructure for full-scale ingredient production just isn’t practical anymore. By partnering with contract development and manufacturing organizations, or CDMOs, companies can rapidly scale operations, tap into niche expertise, and reduce time to market—all without compromising on safety or efficacy.
How are global supply chain challenges influencing this trend?
The global pandemic, along with ongoing geopolitical tensions, exposed critical vulnerabilities in pharmaceutical supply chains. In response, companies are shifting toward more diversified and resilient sourcing strategies. CDMOs offer regional manufacturing capabilities, redundant systems, and supply chain flexibility that pharmaceutical firms now see as essential. This trend echoes what’s also happening in the Immunology Market, where decentralization and rapid adaptation have become survival strategies.
What technological innovations are reshaping this outsourced model?
Modern CDMOs aren’t just service providers—they’re innovation partners. Many are investing in cutting-edge technologies like continuous manufacturing, automation, and advanced analytics. These improvements allow for more precise control, faster production timelines, and better product quality. As regulatory demands evolve and drug formulations become more complex, CDMOs are proving to be not only manufacturing hubs but also RD collaborators.
Is the market growth being led by specific drug categories or regions?
Yes, certain drug classes such as oncology, cardiovascular, and biologics are seeing faster demand due to ongoing medical research and an aging population. North America and Asia-Pacific are leading in terms of CDMO activity, with countries like the US, India, and China emerging as key players. The sheer scale and expertise available in these regions make them preferred hubs for active ingredient production and development.
How are regulations affecting the expansion of this outsourcing model?
CDMOs are under constant scrutiny from regulatory bodies like the FDA and EMA, but that hasn’t slowed the market—it’s pushing it toward higher standards. Top-tier CDMOs are adopting Good Manufacturing Practices (GMP) and building audit-ready facilities to attract major clients. Regulatory compliance has become a competitive advantage, not just a requirement.
What are the key challenges companies face when outsourcing to CDMOs?
Despite its advantages, outsourcing API production isn’t without hurdles. Intellectual property protection, communication gaps, and dependency on third-party timelines can complicate things. However, many of these challenges are being mitigated by long-term partnerships, digital project management tools, and improved transparency across the board.
Are we seeing any major investments or acquisitions in this space?
Absolutely. The race to capture market share has triggered a wave of mergers, acquisitions, and billion-dollar investments. Large pharmaceutical companies are either acquiring CDMOs or locking in multi-year agreements to secure production capacity. The strategic focus is clear—control critical supply chains, reduce risk, and stay ahead of the innovation curve.
What does the future hold for this fast-evolving sector?
The Active Pharmaceutical Ingredient CDMO Market is poised for long-term growth as the global healthcare landscape becomes more dynamic and decentralized. With personalized medicine and biologics on the rise, demand for specialized manufacturing partners will only grow. This is no longer a behind-the-scenes support industry—it’s becoming the backbone of modern pharmaceutical innovation.
For companies aiming to move fast, stay lean, and outpace competitors in drug development, partnering with an experienced CDMO might just be the smartest move they can make.